Audit and Inspection Readiness
Create and maintain an ongoing state of Inspection Readiness (IR) across your organization
Understand areas of risk and take practical steps to remediate them. Establish a state of inspection readiness and put the oversight in place to ensure you can demonstrate a compliant operation and to support the management and follow up of Health Authority inspections.
TriRadial provides expertise, services and tools in the following areas of Inspection Readiness:
- IR Assessments with prioritized recommendations for action
- Practice Inspections across GCP, PVP, GLP operations
- Executive Management Key Performance Indicators and dynamic dashboards
- IR Training for internal Subject Matter Experts and clinical site staff and Inspection Management training for your organization
- Remediation Support to quickly address compliance gaps
- Quality Expertise on hand to act as staff augmentation as you build out your team

Inspection Readiness Assessments and Remediation
The first step in establishing a state of effective inspection readiness is to understand where you stand today and where your greatest areas of risk exist. Our global network of seasoned professionals will review your current procedures, outputs, tools, staff and programs. Each TriRadial team member has a minimum of 20 years’ experience interacting with and responding to Health Authority officials. After assessing your areas of risk, we can help you to develop clear corrective action plans to address areas of weakness with minimal disruption to your day-to-day business.
Learn how an emerging biopharma company and a company going thru their first Pre-Approval Inspection (PAI) approached their inspection readiness assessments

Inspection Readiness Training
Your team members understand their roles and they are experts in the science. We can help them understand the inspection process, be better communicators with investigators, and to be more organized during an inspection. We will provide practice scenarios and customized role-play to get them up to speed and build confidence. We will also help you to design and organize an efficient backroom operation so that you can get appropriate evidence to HA investigators in a timely and organized manner.
View how TriRadial helped an emerging biopharma train their team across all GxP's to be prepared for their upcoming inspection


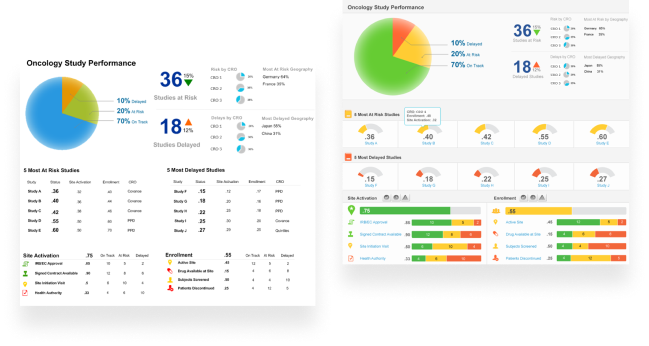
Key Performance Indicators and Dashboarding
As they say, you cannot manage what you cannot measure. Our team has an extensive set of Key Performance Indicators (KPIs) with defined thresholds for action that span all the main areas of your operation. These KPIs allow leaders to have a direct line of sight into operational performance and enable them to manage by exception. With links to your operational systems, the dashboards can be dynamic, drillable and span all key areas of your organization.
Click to download a sample of some of our inspection readiness dashboards.
Mock Inspections
You don’t want to find out how you will perform by undergoing an actual inspection without any practice. We will help you get up to speed by designing and conducting tailored practice sessions prior to a mock inspection or a real one. Complete with “hats on/hats off” sessions to coach and guide you, we will work with your team in both front-room and back-room follow up, to help you to be inspection ready when you need to be.
Learn more about our inspection readiness framework

Inspection Management and Quality Staffing
Your programs are advancing quickly. Your quality infrastructure has not kept pace with your growth. You need short-term, high-quality expertise in place who has been through Health Authority inspections in the past. Sometimes your need to fill key roles outpaces your ability to find the right people. TriRadial has a global network of quality experts with decades of experience, who can quickly step in and bridge the gap until you can fill the roles internally.
