Helping Life Sciences companies navigate growth to bring therapies to patients faster
New York | Cambridge | Zug | Paris
Helping Life Sciences companies navigate growth to bring therapies to patients faster
New York | Cambridge | Zug | Paris
We are solely focused on the Life Sciences industry
We provide deep domain expertise to help biopharma and medical device companies navigate scalable growth globally
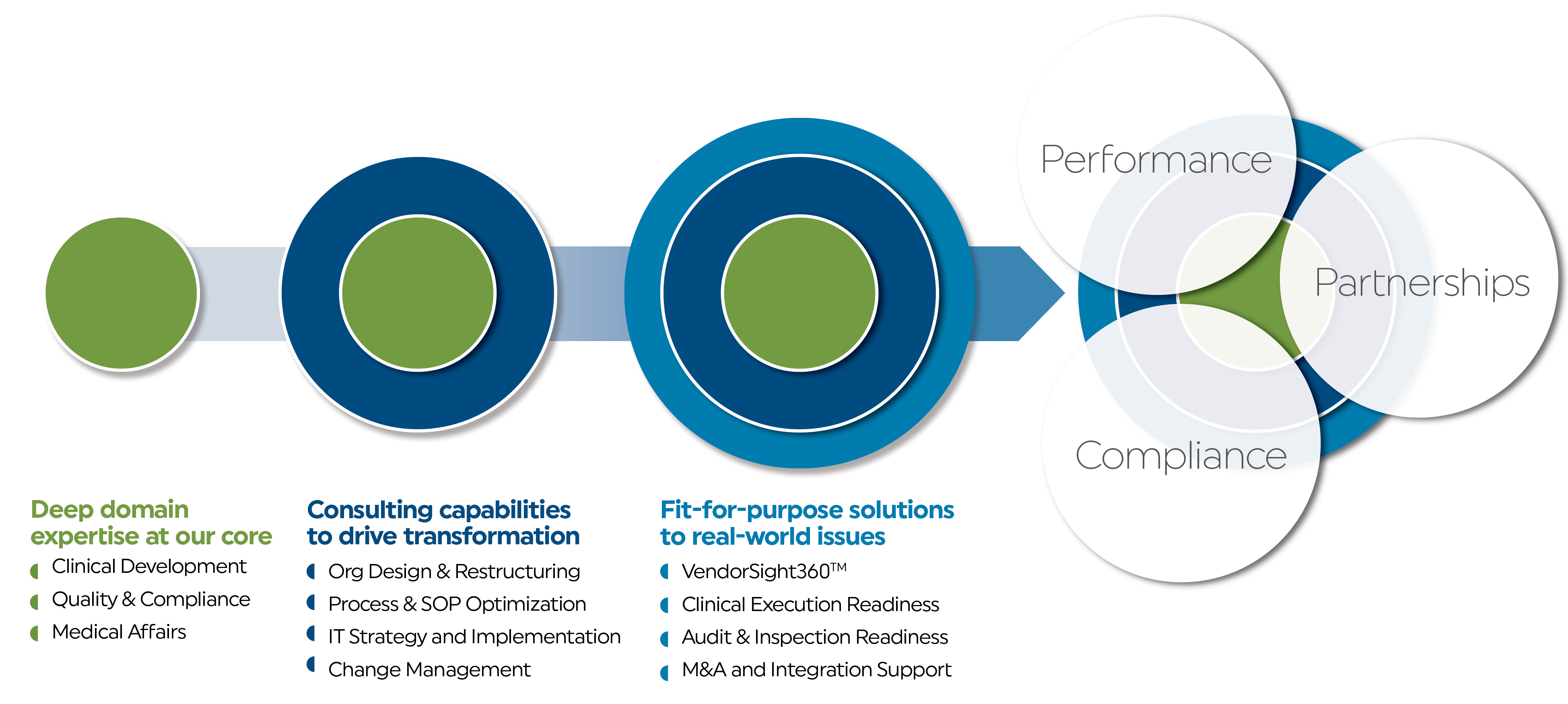
Clinical Development
Quality & Compliance
Medical Affairs
We help emerging and mid-market life sciences companies accelerate growth
We help rapidly growing biopharma companies build the internal capabilities needed to scale globally and drive the best outcomes from vendors
We leverage deep domain expertise in each of our solutions to help companies scale globally
There is no substitute for
real-world global experience
Below are examples of challenges our clients have experienced across the R&D space
| Clinical | Quality | Regulatory | Safety/PV | |
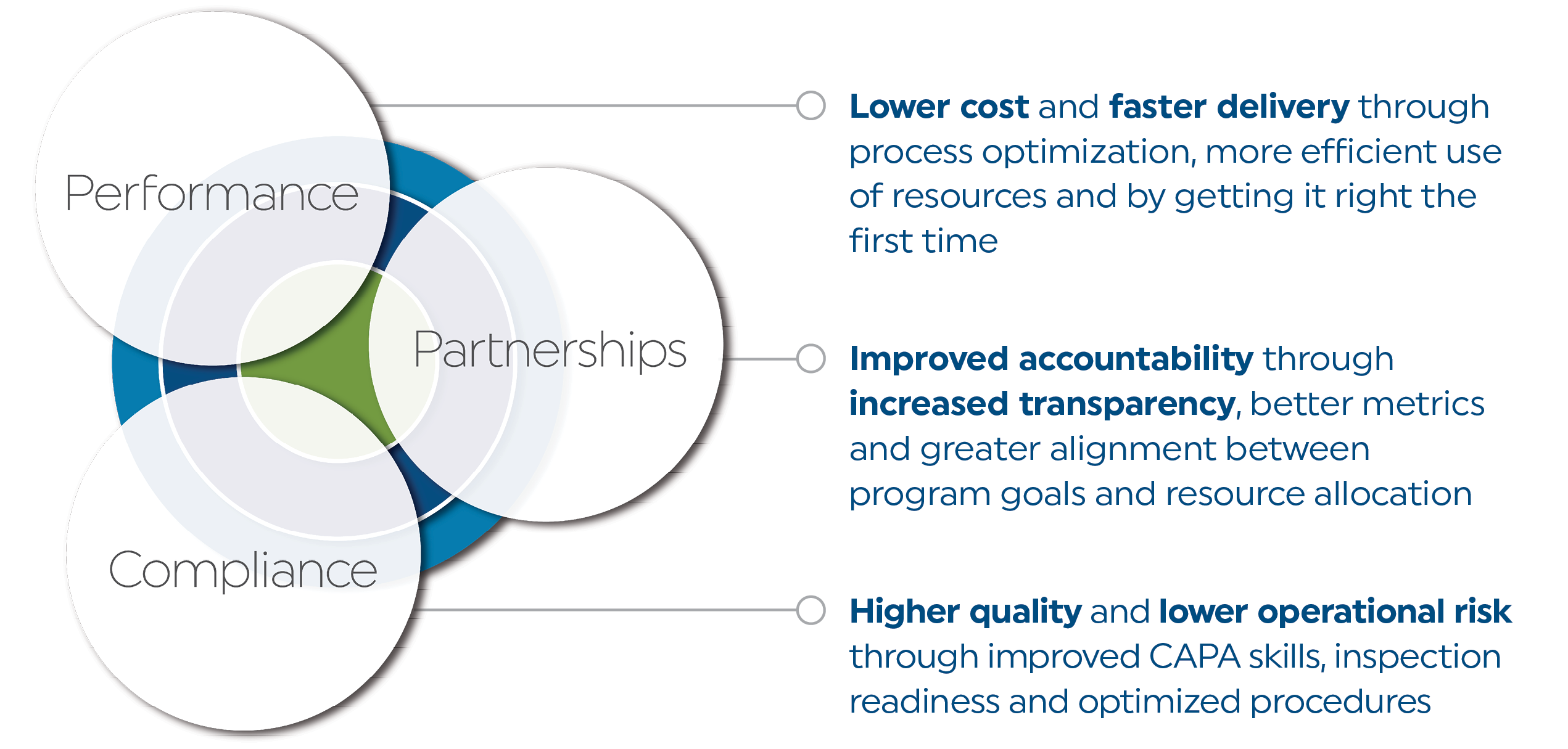
| Performance |
|
|
|
|
| Partnerships |
|
|
|
|
| Compliance |
|
|
|
|

We develop implementable strategies and practical solutions that are based on reality, not theoretical assumptions
Click on the links below to explore a few of the solutions and capabilities we offer to help you tackle complex issues and transform your operational performance















GCP Quality Inspection Readiness Assessment
A rapidly growing pre-commercialization biotech company has outpaced its ability to demonstrate effective quality oversight of its GCP activities. It needs a detailed review of its Clinical organization and Quality Management System including core processes, technology, and organizational structure. It specifically needs to review its capabilities in the face of ICH E6 R2 requirements for risk-based methods.
PV Inspection Readiness Assessment
A biotech company has just received its first commercial product approval. Anticipating an inspection, new leader of its Pharmacovigilance organization needs a quick and comprehensive assessment of current gaps, potential risks and ideas for tool improvements. Given that operational activities are largely outsourced to a vendor, its ability to demonstrate oversight of vendor activities is a critical to its inspection readiness under ICH E6 R2.
GxP Inspection Readiness Training
A fast-growing biopharma company has just been granted approval for its first commercial product. Most of the client’s team has never experienced a full non-GMP inspection. They completed a gap assessment and are actively addressing findings. But the entire organization needs training and guidance on hosting the Health Authority and presenting itself as organized, competent and capable of answering any question that may arise with speed and professional calm.
Clinical Process Transformation
A mid-sized biotech company was focused on executing a global clinical pipeline but, like many emerging companies, had inefficient and disjointed processes. In addition, processes and teams were not fully grounded in GCP or other regulatory compliance needs. These challenges combined to make the development process cumbersome, inefficient and open to compliance risks.
Clinical Project Management Org and Process
A small, rapidly growing Biotech had several therapeutic assets moving to clinical development. It faced challenges in strategic development decisions, CRO negotiations, and senior management information reporting. It needed a consistent means by which to manage and report, and to focus on the science without being encumbered by complicated tools. Timing was critical as some team members would soon be on leave and the company had yet to achieve its first approval.
Sourcing Strategy
A mid-sized biopharma company needed to identify internal activities that should be outsourced and implement an objective, defendable process to select preferred vendors that not only fit specific functional/study needs, but also represent best value for investment.
Vendor Selection Process
An emerging biopharma company had an inefficient and disjointed process for the selection of vendors to support study-specific activities, from service need identification through contract execution. They had difficulty aligning on needs and during the bidding process, leading to challenging bid comparisons and an over-reliance on Start-Up Agreements (SUAs)
Procurement and Vendor Management Staffing
A small biotech company did not have internal resources or capabilities to support Procurement and Clinical Vendor Management functions; the internal team was focused primarily on Study Design and Clinical Operations execution. These gaps in key business functions, coupled with a rapidly expanding portfolio, presented significant risks to timelines and the ability to manage critical vendor activities.
Vendor Governance and Oversight
A rapidly growing pre-commercial biotech company found it increasingly difficult to manage large-scale clinical trials being executed by two costly global CROs. The company needed a robust oversight process to handle the rapidly increasing volume of planned trials, since consistent processes, metrics and tool to ensure on-time and high-quality delivery did not exist.
R&D Merger Integration
A rapidly-growing EU-based biotech company purchased a US-based entity. The company needed assistance planning and leading integration activities to harmonize across all Development functions. Some of the capabilities between the two entities were complementary, but they were interested in rapid integration globally, to align with their newly-developed strategy.
Contracting Process
A small growing Biotech was bringing its therapeutic assets to full clinical development. Balancing the need to move through clinical development in a safe but agile manner while guarding the company’s assets and leveraging emerging relationships with strategic suppliers was proving a difficult balance, particularly as different stakeholders had distinct needs and perspectives. The result was a protracted contracting process which generated confusion and slowed work progress. All of this while ensuring suppliers met quality and compliance standards to ensure the validity of clinical results.
CRO Rescue
A biotech company with products in early clinical phases sought to reduce its spend with a large Contract Research Organization, while looking for options to replace with a lower-cost and more responsive CRO. The internal team has limited experience interacting with CROs and needs guidance to ensure a successful trial outcome.
Change Order Evaluation and Negotiation
A small biopharma company was expecting a large Change Order (CO) from it’s primary CRO on a Phase IIB global clinical program that was experiencing delays. The company had limited means by which to evaluate the CO, given a mix of performance issues and COVID-related delays in study start-up and recruitment, as well as cost overruns due to low initial estimates for investigator pass-through grants.
Data Integrity Review
A global genomics data company is building out its core processes and go to market strategy. It must recognize and integrate key quality principles to reduce operational and compliance risk. It conducts its operations in Europe, Asia and North America and is subject to Data Privacy regulations across all jurisdictions. The goal is to make its processes robust and compliant everywhere it does business.
Invoice Accrual Process
Leading biotech customer with a growing pipeline, relying more heavily on outsourcing partners, needs a way to effectively manage and control portfolio spend and obtain clarity related to study level financials to inform business decisions.









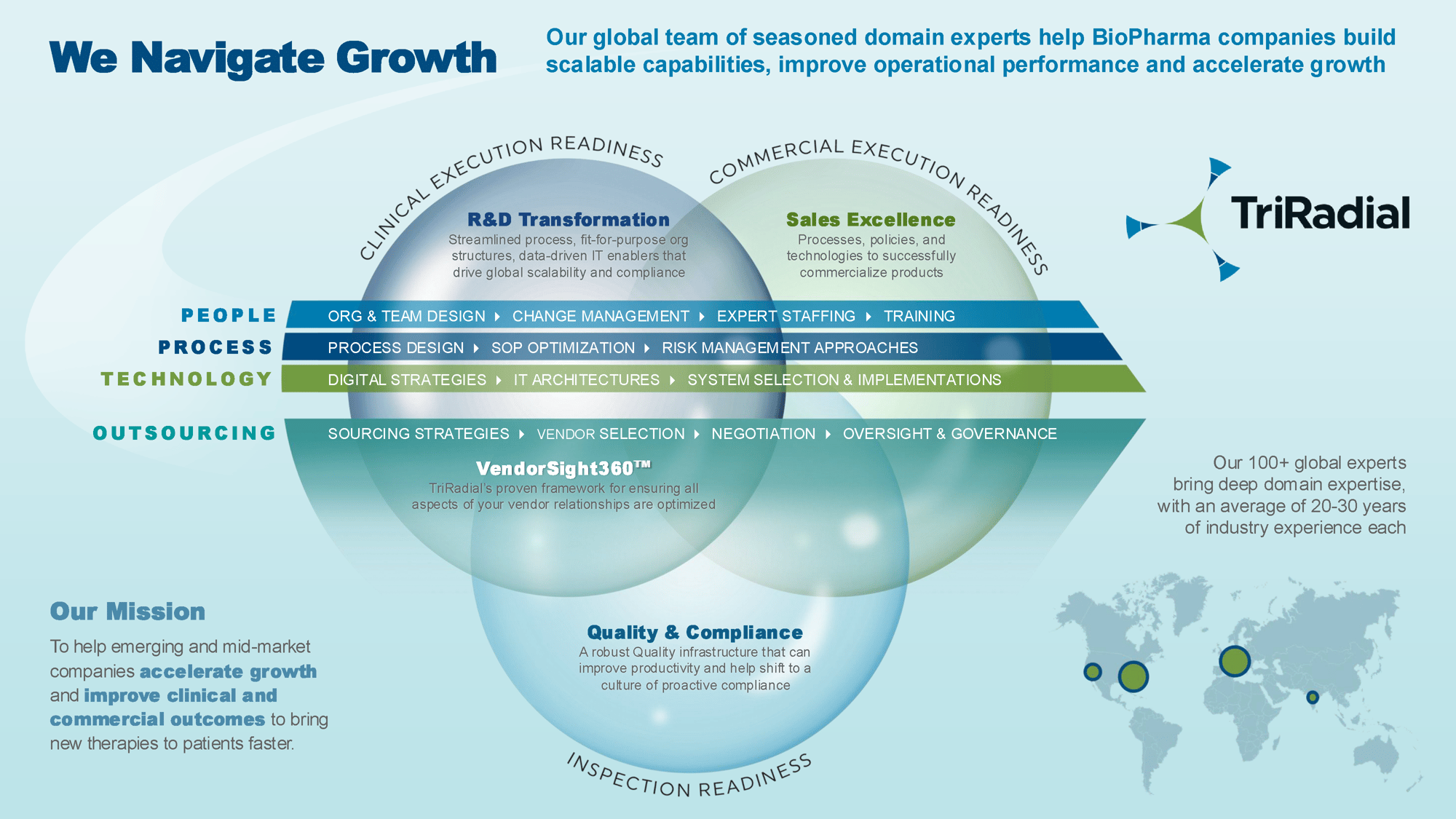
VendorSight360™
VendorSight360™ is TriRadial’s proven methodology for ensuring all aspects of your vendor relationship are optimized. VendorSight360™ ensures effective oversight in all stages of the vendor cycle, which is essential to maximizing performance from your vendors while improving transparency and managing risk.
Clinical Execution Readiness
Ensure that your organization has the appropriate people, processes, procedures, partners, and systems in place to achieve successful clinical outcomes while avoiding delays and roadblocks that have become all too common in clinical research.
M&A Integrations and Divestitures
Leveraging our combined experiences, TriRadial’s M&A experts have developed a highly customizable and flexible framework and a comprehensive Integration Planning & Execution Playbook.
Commercial Execution Readiness
It can be a long journey on the path to commercialization of a product, but once there, how you establish and operate the commercial capabilities of your company can ensure their success or cause you to fail in reaching the full potential of that product.
Architecting a Scalable and Proactive Quality Infrastructure
A scalable Quality infrastructure can improve productivity, create a competitive advantage and help shift a company’s culture to one of proactive compliance
Ensuring Effective and Compliant Vendor Oversight in an Era of Increased Outsourcing
The need to demonstrate full awareness and control of key vendor activities and outputs is becoming more critical as outsourcing continues to increase.
Building World Class CAPA Skills
A structured CAPA framework can make the difference between continually fighting the same fires and solving problems effectively once to create sustainable solutions.
Process-driven compliance, right-sized for your organization
A streamlined, compliant process is only useful if the organization is able to implement it. Processes and SOPs should balance performance and regulatory compliance.
Developing a Proactive Framework for Audit and Inspection Readiness
A well-run inspection or audit, supported by a sustainable, integrated framework, can help reduce the severity of a finding or sometimes avert one altogether.

The TriRadial Team
TriRadial Solutions is an advisory firm committed to delivering practical, high-quality People, Process and Technology solutions to help your business grow. Our team of industry experts and seasoned management consultants can help assess where you are currently and navigate a path forward to building robust, integrated capabilities poised for scalable growth.

The TriRadial team is ready to answer your questions
For more information on any of our solution offerings or questions about how we can help you transform your business, please e-mail us using the form to the right, or schedule a meeting with Geoff Garabedian below or feel free to call us anytime.










